Using high-entropy materials, the researchers created more efficient thermoelectric materials than previously possible, an advancement that they said could even help make long-distance space exploration possible. They published their results in the journal Joule.
Thermoelectric devices—including the radioisotope thermoelectric generators that produce energy for NASA's space exploration vehicles—can convert differences in temperature to electricity. When they are placed near a heat source—like a steam pipe in a power plant—charge carriers, like electrons, move from the hot side to the cold side, producing an electric current.
Current commercially available devices boast 5% to 6% efficiency. The researchers used their new fabrication approach to create a prototype that reached 15% conversion efficiency. The improved efficiency means that existing devices could shrink by 200% and still produce the same energy, or same-sized device could produce 200% energy, the researchers said.
"These findings show a new direction in how we can improve thermoelectric devices to be really efficient," said Bed Poudel, research professor in the Department of Materials Science and Engineering at Penn State and co-author on the study. "Our work provides a new avenue toward creating very exciting thermoelectric materials and could lead to even greater advances with future material development."
The improved efficiency means that existing devices could shrink by 200% and still produce the same energy, or same-sized device could produce 200% energy.
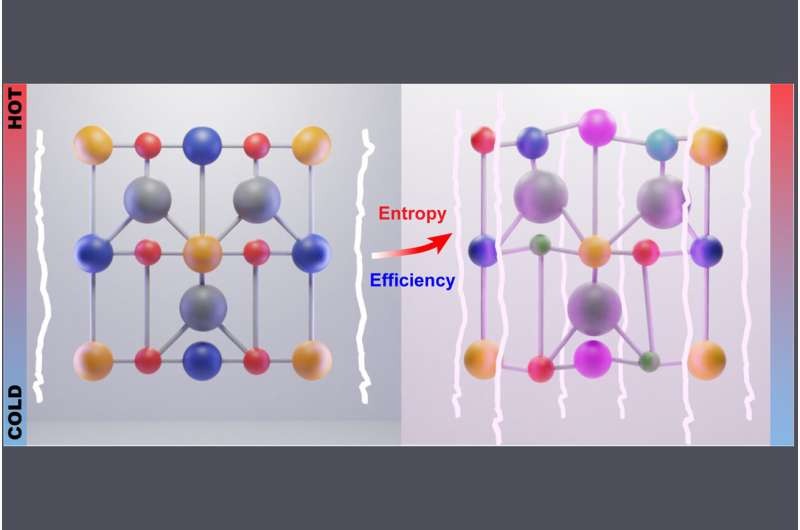
The Penn State team previously used half-Heusler alloys —a special class of materials that are good at generating thermoelectric power at medium-high temperatures—to improve device performance. These materials are typically alloys made of three metallic elements, sometimes with dopants, or small amounts of other materials, added to boost performance.
In the new work, the scientists turned to high-entropy half-Heusler materials. These alloys, which are made of at least five principal elements in a single crystalline structure, boast the same properties found in half-Heusler materials but enhanced.
"What we did in this work was successfully integrate high-entropy engineering into a half-Heusler system," said Wenjie Li, associate research professor at Penn State and a co-corresponding author of the study.
"With conventional compounds, you may have 100 options to make different chemical compositions. But when we use the high-entropy concept, we can make maybe thousands of chemical compositions in order to alter the material properties."
The scientists said using high-entropy materials with more atoms means the crystalline structures are more disordered and charge carriers take longer to move through the material, lowering its thermal conductivity. The additional atoms are selected in such a way that the material maintains a higher power factor, a measure of how efficiently an electrical system can convert power into useful work.
"In this concept, we can simultaneously maintain a high-power factor and get a low thermal conductivity to maximize the figure of merit, which is a measure of the materials' effectiveness," said Subrata Ghosh, a postdoctoral scholar at Penn State and lead author of the study.
"High-entropy engineering can be incorporated with conventional approaches to improve the figure of merit further in any class of thermoelectric materials."'
The new thermoelectric material achieved a record high figure of merit of 1.50 at a temperature change of 1,060 degrees Kelvin, or roughly 1,448 degrees Fahrenheit. That represents a 50% increase from current cutting-edge materials, the scientists said.
"High-entropy materials are often used in high-temperature refractory applications like jet engines or hypersonic vehicles, but this is the first time they have been used to develop a superior half-Heusler thermoelectric system," Li said.
The work has implications for creating more efficient devices for waste heat recovery in industrial settings. Recovering this waste heat and using it to provide electricity can reduce fossil fuel consumption. And because they have no moving parts and produce no chemical reactions or emissions, thermoelectric devices offer a promising source of clean energy, the scientists said.
Thermoelectric devices resemble a table with two legs—one leg made of p-type and one of n-type semiconductor material. The current study only applies to the p-type material, and the scientists said further work to apply this to the n-type could result in additional increases in efficiency.
"If we can implement this to a wider class of thermoelectric materials and continue getting good figures of merit, we can really push the conversion efficiency toward 20% or more," Poudel said.
"That would be very competitive with solar energy or other technologies for solid state power generation. That is the exciting part of it—to see what this can lead to in future material development."
According to TechXplore
