Tuesday, 03/02/2026 | 14:29 GMT+7
The discovery is an important development in the worldwide effort to mimic the way plants make fuel from sunlight, a key step in creating a green energy economy. It was reported last week in Nature Materials by theorist Jens Norskov of the Department of Energy's SLAC National Accelerator Laboratory and Stanford University and a team of colleagues led by Ib Chorkendorff and Soren Dahl at the Technical University of Denmark (DTU).
Hydrogen is an energy dense and clean fuel, which upon combustion releases only water. Today, most hydrogen is produced from natural gas which results in large CO2-emissions.
![]()
An alternative, clean method is to make hydrogen fuel from sunlight and water. The process is called photo-electrochemical, or PEC, water splitting. When sun hits the PEC cell, the solar energy is absorbed and used for splitting water molecules into its components, hydrogen and oxygen.
Progress has so far been limited in part by a lack of cheap catalysts that can speed up the generation of hydrogen and oxygen. A vital part of the American-Danish effort was combining theory and advanced computation with synthesis and testing to accelerate the process of identifying new catalysts.
This is a new development in a field that has historically relied on trial and error. "If we can find new ways of rationally designing catalysts, we can speed up the development of new catalytic materials enormously," Norskov said.
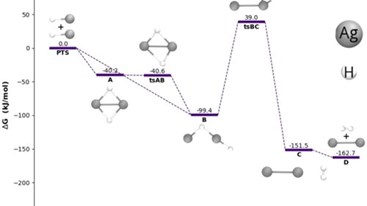
The team first tackled the hydrogen half of the problem. The DTU researchers created a device to harvest the energy from part of the solar spectrum and used it to power the conversion of single hydrogen ions into hydrogen gas. However, the process requires a catalyst to facilitate the reaction.
Platinum is already known as an efficient catalyst, but platinum is too rare and too expensive for widespread use. So the collaborators turned to nature for inspiration.
They investigated hydrogen producing enzymes-natural catalysts-from certain organisms, using a theoretical approach Norskov's group has been developing to describe catalyst behavior.
"We did the calculations," Norskov explained, "and found out why these enzymes work as well as they do." These studies led them to related compounds, which eventually took them to molybdenum sulfide. "Molybdenum is an inexpensive solution" for catalyzing hydrogen production, Chorkendorff said.
The team also optimized parts of the device, introducing a "chemical solar cell" designed to capture as much solar energy as possible. The experimental researchers at DTU designed light absorbers that consist of silicon arranged in closely packed pillars, and dotted the pillars with tiny clusters of the molybdenum sulfide. When they exposed the pillars to light, hydrogen gas bubbled up-as quickly as if they'd used costly platinum.
The hydrogen gas-generating device is only half of a full photo-electrochemical cell. The other half of the PEC would generate oxygen gas from the water; though hydrogen gas is the goal, without the simultaneous generation of oxygen, the whole PEC cell shuts down.
Many groups-including Chorkendorff, Dahl and Norskov and their colleagues-are working on finding catalysts and sunlight absorbers to do this well. "This is the most difficult half of the problem, and we are attacking this in the same way as we attacked the hydrogen side," Dahl said.
Norskov looks forward to solving that problem as well. "A sustainable energy choice that no one can afford is not sustainable at all," he said. "I hope this approach will enable us to choose a truly sustainable fuel."
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, California, SLAC is operated by Stanford University for the U.S. Department of Energy Office of Science.
SUNCAT is a DOE Office of Science-sponsored research center at SLAC in partnership with the Department of Chemical Engineering, Stanford University, to explore catalytic processes for energy conversion and efficiency.
The Technical University of Denmark, DTU is a technical university in northern Europe. The research focus is on technical and natural sciences such as catalysis, photonics, wind energy, biotechnology and telecommunication.
The Center for Individual Nanoparticle Functionality, CINF, is funded by the Danish National Research foundation and is focusing on nanoparticle functionality in conjunction with energy harvesting, conversion and production.
Catalysis for Sustainable Energy, CASE, is a cross disciplinary initiative funded by the Danish Ministry of Science. The goal is to develop rules of catalyst design and use these rules to design cheap, efficient and stable catalysts for converting solar energy into fuels.
solardaily.com